ABC Transporters Interacting Proteins: Regulation Through Protein Interactions
ABC Protein Interactions - Proteins rarely, if at all, function alone or independent of other proteins. Proteins functions are generally regulated by post-translational modifications that modulate their interactions with other protein, altering their sub-cellular localizations, half-lives and interacting partners.
ABC Transporters - Are a class of transmembrane proteins containing an ATP Binding Cassettes (ABC) consensus sequence. ABC proteins are active transporters evolutionary conserved from bacteria to man. Humans encode 48 members divided into seven subfamilies (ABCA to ABCH). Modulating ABC transporters expression can lead to major phenotypic changes in normal and diseased tissues and organs.
Given the important functions of ABC transporters in mediating normal and disease cell physiologies, we believe ABC transporters' functions are regulated by protein-protein interactions. Our immediate focus is on identifying ABC transporters interacting proteins and their interacting domains.
Functional ABC transporters encode two cytoplasmic domains, a cytoplasmic nucleotide binding domain(s) (e.g., NBDs, each containing one ABC signature sequence) and two transmembrane domains (e.g., TMDs, each encoding six transmembrane helices). Some ABC transporters contain an additional domain termed, Linker Domain(s) (e.g., L1, or L0 and L1). The NBDs are thought to energize the transport functions of ABC proteins, through conformational changes of the TMD helices, the TMDs are believed to encode their ligand binding sites and determines their substrate specificities. The function(s) of the linker domains remains a matter of speculation and is currently unknown. Interestingly, while the NBD shares the highest sequence identity between different ABC transporters and across different species, the Linker domains share NO sequence identity, are highly charges and encode several phosphorylation sites. Moreover, although high-resolution crystal structure of several ABC transporters have been resolved, it has NOT been possible to determine the structure of the linker domain as it encodes disordered structure. It is interesting to note that disordered structures are normally involved in protein-protein interactions, whereby such protein interactions are modulated by post-translational modification.
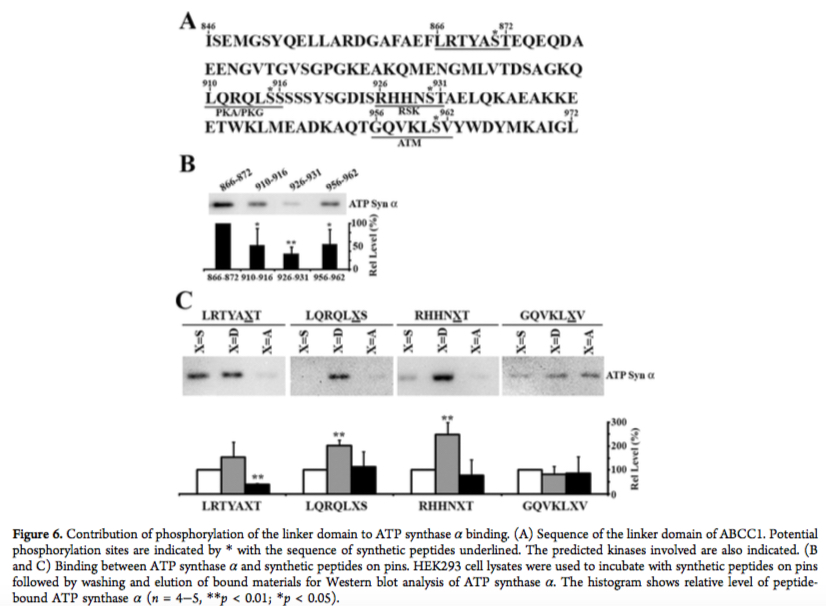
In one example, we have identified specific sequences in ABCC1 linker domain that bind with high affinity to tubulin dimer. Phosphorylation of these interacting sites inhibited their binding to tubulin. Interestingly, the same sites in ABCC1 linker domain interact with ATP-synthase alpha, but only when these sites are phosphorylated.
It will be of interest to know if the same domains of other ABC transporters also bind to the same or different proteins. Moreover, how these protein interactions affect their sub-cellular localization, half-lives and functions.
Figure 1
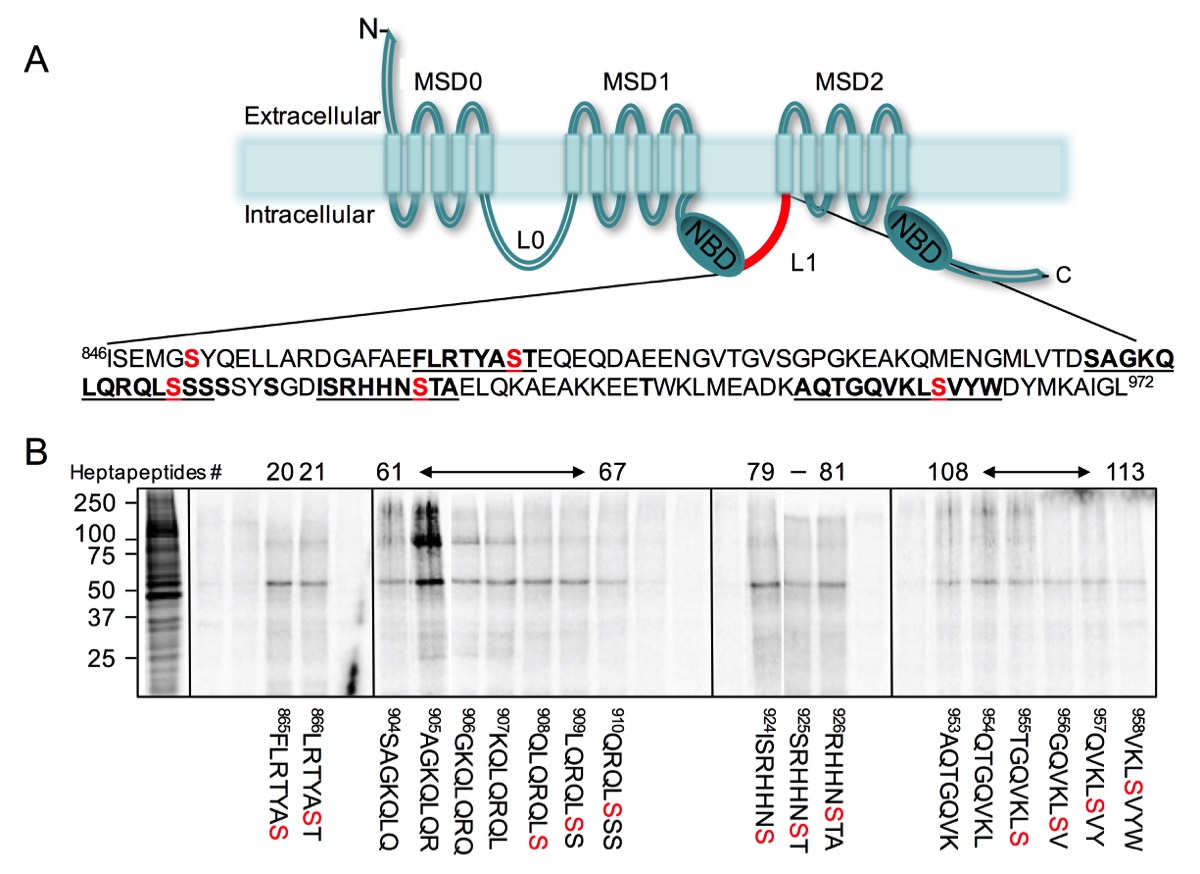
Figure 2
Our Experimental Approaches
Project 1 - Identify ABCB1, C1, G1 and G2 interacting proteins using scanning peptide approach, followed by MS-MS. Map the above interacting sequences on ABC-transporters X-ray resolved structures. Confirm the identity of the interacting proteins using biochemical, photo-reactive amino acids and confocal microscopy. Determine the effects of Post-translational modifications on ABCB1, C1, G1 and G2 on their interactions.
Project 2 - Determine ABC transporters' interacting proteins in drug-sensitive and (selected) drug-resistant cells using Bir A-fusion constructs. Combine all three protein-interaction approaches and their supporting biochemical assays to examine the effects of cellular perturbation on ABC transporters' interactions and protein environment. Determine the functional roles of the interacting proteins on ABCB1, C1, G1 and G2, using CRISPR knockout approach.
Project 3 - Examine the effects of Phosphorylation sites on the Linker conformation, using spectroscopy and NMR. Determine the effects of different ABCB1, C1, G1 and G2-Phosphorylation modified mutants on their drug/ligand transport. Study the effects of switching Linker between compatible ABC transporters and determine effects on functions.
Peer-reviewed manuscripts on ABC Interacting Proteins
Sequences in Linker-1 domain of the multidrug resistance associated protein (MRP1 or ABCC1) bind to tubulin and their binding is modulated by phosphorylation. Ambadipudi R, Georges E. Biochem Biophys Res Commun. 2017 Jan 22;482(4):1001-1006.
Human ABCC1 Interacts and Colocalizes with ATP Synthase α, Revealed by Interactive Proteomics Analysis. Yang Y, Li Z, Mo W, Ambadipudi R, Arnold RJ, Hrncirova P, Novotny MV, Georges E, Zhang JT. J Proteome Res. 2012 Feb 3;11(2):1364-72.
The P-glycoprotein (ABCB1) linker domain encodes high-affinity binding sequences to alpha- and beta-tubulins. Georges E. Biochemistry. 2007 Jun 26;46(25):7337-42.
ABCG2 membrane transporter in mature human erythrocytes is exclusively homodimer.
Leimanis ML, Georges E. Biochem Biophys Res Commun. 2007 Mar 9;354(2):345-50.