Malaria Drug Resistance
The rise and spread of resistant parasites (Plasmodium falciparum) is a major obstacle in the treatment of infected patients. Mutations in membrane transporters (e.g., P-glycoprotein homolog (P-gh1) and the Chloroquine Resistance Transporter (CRT)) have been shown to cause drug resistance to various antimalarials, as most of current drugs appear to target the parasite's digestive vacuole. The normal ligands transported by P-gh1 and PfCRT have not been definitively demonstrated; but PfCRT may transport glutathione, short peptides and aromatic amino acids. It is currently well accepted that mutations in pfcrt and pfmdr1 genes are causative of chloroquine and mefloquine resistance, respectively. Moreover, P-gh1 appears to attenuate PfCRT-mediate chloroquine resistance.
Malaria Drug Resistance - Membrane transporters expressed at the digestive vacuole (DV) have been shown to confer resistance to several current antimalarials. Research over the past several decades have shown that mutations in P-gh1 and PfCRT are causative of drug resistance to structurally diverse drugs. Moreover, it has been shown that both P-gh1 and PfCRT have overlapping substrate specificities. However, while one transporter (P-gh1) transports drugs into the DV from the cytosol, the other transporter (PfCRT) transports drugs from the DV into the parasite cytosol. This latter point, together with overlap in substrate specificities of the two transporters, whereby toxic drugs (e.g., MK571, a quinoline-based drug) that are better substrates for P-gh1 but block PfCRT function can be used to target drug resistant parasites (see Figure 1).
In addition, give the essential physiologic roles of these membrane transporters (e.g., P-gh1 and PfCRT) for the parasite's survival and proliferation. The normal functions of P-gh1 or PfCRT, and their endogenous ligands, would expand potential of developing yet other novel antimalarials that target their normal functions.
Our research focuses on the molecular mechanisms of these transporters in antimalarials drug resistance and normal physiological functions. With regards to the latter we focusing on understanding the biochemistry and regulation of PfCRT.
Figure 1
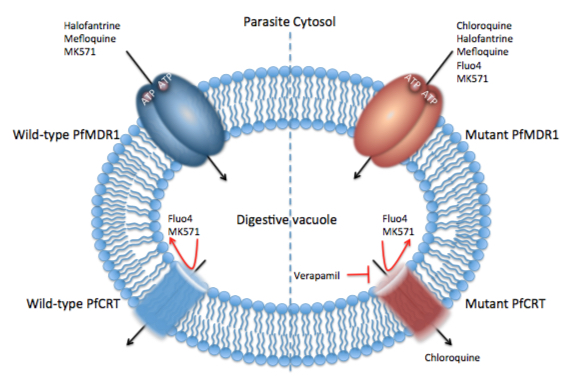
Figure 2
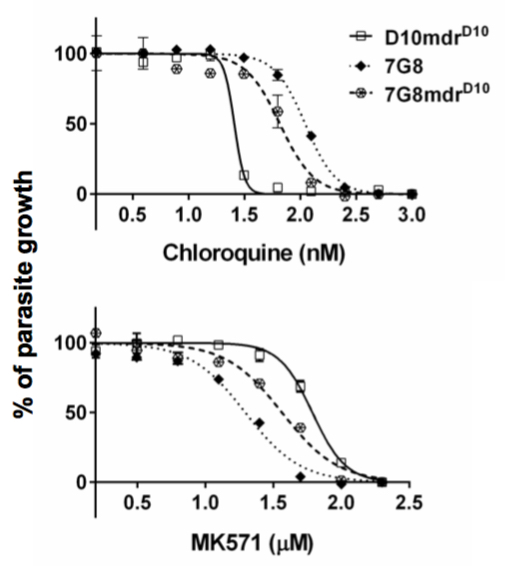
Our Experimental Approaches
Project 1 - Study the biochemistry and structure of PfCRT in P. falciparum and heterologous expression system. Identify PfCRT interacting proteins in P. falciparum. Identify the endogenous ligands transported by P-gh1 and PfCRT.
Project 2 - Determine the mechanism by which MK571 interact with P-gh1 and PfCRT and use this knowledge and tool to isolate more potent MK571-like drugs that can be advanced to clinical phases. Such drugs would be selectively toxic to drug resistant P. falciparum.
Project 3 - Study the structure and function of PfABCG in P. falciparum. Determine the normal substrate for PfABCG and confirm its role as drug and/or sterol transporter (i.e., more like human ABCG2 or ABCG1).
Peer-reviewed manuscripts on Malaria Drug Resistance
3-Iodo-4-aminoquinoline derivative sensitises resistant strains of Plasmodium falciparum to chloroquine. Edaye S, Tazoo D, Bohle DS, Georges E. Int J Antimicrob Agents. 2016 Jun;47(6):482-5.
3-Halo Chloroquine Derivatives Overcome Plasmodium falciparum Chloroquine Resistance Transporter-Mediated Drug Resistance in P. falciparum. Edaye S, Tazoo D, Bohle DS, Georges E. Antimicrob Agents Chemother. 2015 Dec;59(12):7891-3.
Characterization of native PfABCG protein in Plasmodium falciparum. Edaye S, Georges E. Biochem Pharmacol. 2015 Sep 15;97(2):137-46.
A 2-amino quinoline, 5-(3-(2-(7-chloroquinolin-2-yl)ethenyl)phenyl)-8-dimethylcarbamyl-4,6-dithiaoctanoic acid, interacts with PfMDR1 and inhibits its drug transport in Plasmodium falciparum. Edaye S, Reiling SJ, Leimanis ML, Wunderlich J, Rohrbach P, Georges E. Mol Biochem Parasitol. 2014 Jun;195(1):34-42.
Lupeol long-chain fatty acid esters with antimalarial activity from Holarrhena floribunda.
Fotie J, Bohle DS, Leimanis ML, Georges E, Rukunga G, Nkengfack AE. J Nat Prod. 2006 Jan;69(1):62-7.
Pleiotropic resistance to diverse antimalarials in actinomycin D-resistant Plasmodium falciparum. Abrahem A, Certad G, Pan X, Georges E. Biochem Pharmacol. 2000 May 1;59(9):1123-32.
Cloning and partial characterization of the proteasome S4 ATPase from Plasmodium falciparum. Certad G, Abrahem A, Georges E. Exp Parasitol. 1999 Nov;93(3):123-31.
Direct binding of chloroquine to the multidrug resistance protein (MRP): possible role for MRP in chloroquine drug transport and resistance in tumor cells. Vezmar M, Georges E. Biochem Pharmacol. 1998 Sep 15;56(6):733-42.